Cell Culture
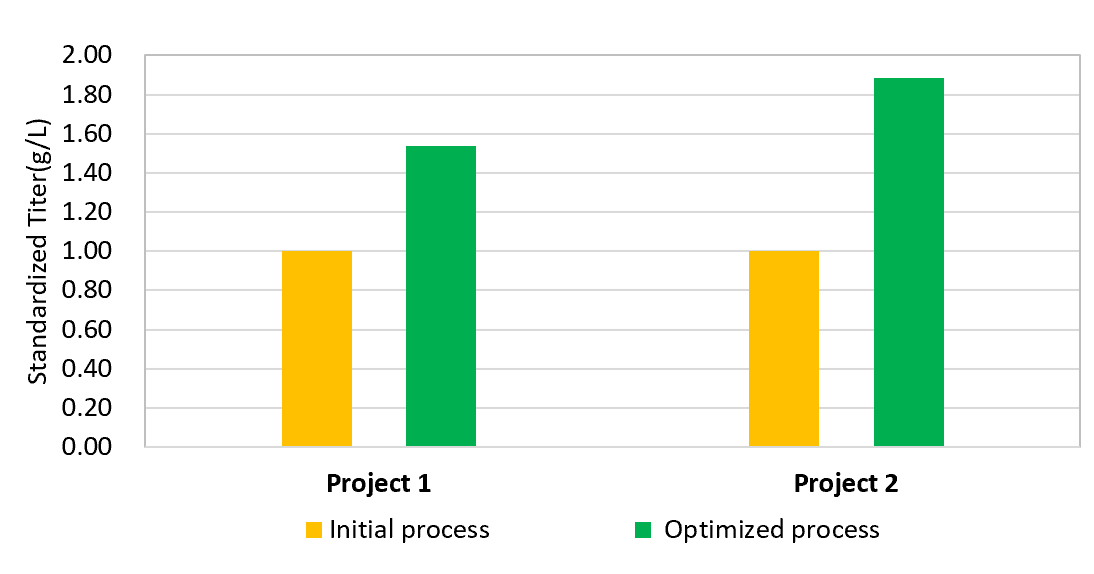
Segregated cell culture process development laboratories provide capability to identify top cell pools and clones, screen media, and optimize product expression and key quality attributes with the capacity to execute up to 4 upstream development programs in parallel.
Our Cell Culture Process development team has broad experience in different types of biologics. We utilize our experts’ deep process development skills, high- standard facilities, and rational experimental design, to establish robust, high-quality and low-cost cell culture processes covering every stage of biologics development.