

Several options for fully traceable expression systems with global freedom to operate. Constructed multiple high-expression stable cell lines for mAbs, bsAbs, and fusion proteins.
Flexible process development services to transfer, develop, scale-up, and optimize customer-specific processes or optimize our proven in-house platform processes.
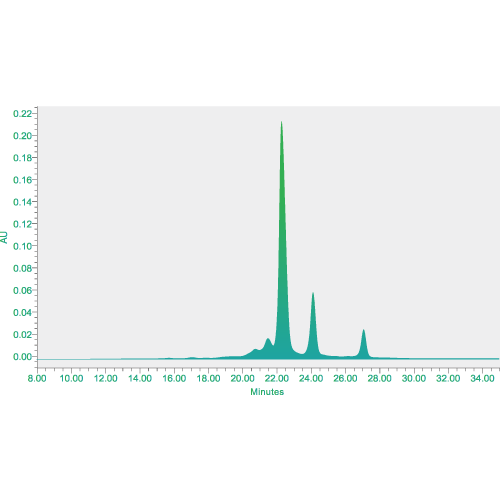
Analytical services for physical and chemical analysis, biological assays, impurity measurement, safety tests and other analytical method development including structural characterization.


Our formulation development platform can meet the different formulation development needs of a broad range of biologic products.


Bioworkshops has rich experience in PC/PV, applying product life cycle QbD concepts to provide CMC services for late-stage clinical products.

Aseptic filling of vials (liquid+lyophilized), prefilled syringes and cartridges by aseptic manufacturing.
With state-of-the-art single use bioprocessing facilities, Bioworkshops brings biologic products to the market quickly with high quality.


Bioworkshops Regulatory Affairs services focus on the CMC documents and drug registration of biologics in China, US and EU.
Bioworkshops is a professional biologics contract development and manufacturing organization (CDMO). We are committed to offering cost-effective and efficient outsourcing solutions to assist our clients shorten the time to start clinical trials and enter markets. Our scope of services includes cell line development, process development, analytical development, formulation development, cGMP manufacturing of drug substance and sterile drug product. Our Mission is working with global partners to achieve rapid approval of clinical and commercial biologic products by applying our expertise in development and manufacturing.
The company owns a 28,000 m2 building and land in Suzhou, China and has invested over US$50 million to build state-of-the-art development and manufacturing facilities.

20 million units/year
Vials (liquid and lyophilization)100 + batches
cGMP manufacturing of drug substance including 2000L13000 L
Total cell culture capacity with 200L, 500L and 2000L single-use bioreactors300 + batches
Aseptic drug product batches manufactured
NMPA Drug Manufacturing License and passed EU QP and FDA cGMP compliance audits. Bioworkshops Pharmaceutical Quality System operates on a fully validated electronic document management system to achieve international GMP compliance. Qualified vendors and material management systems assure multi-compendial materials and product quality.
Combining strong team, high-capacity, robust supply chain, and full service from cell line development through to drug product, Bioworkshops actively manage completion of all deliverables on time and fulfilling all regulatory requirements.
Lead or contributed to the development of at least 60 antibody products, with many now advanced to late-stage clinical trials and some on market. Deep practical experience covering the full lifecycle of biologic products from process development and pilot production to commercial manufacturing. Effective teamwork.
Multiple platforms of single-use bioreactors and aseptic filling lines which span the requirements of cGMP manufacturing of clinical and large-scale drug substance and aseptic drug products, with high flexibility and production efficiency.
Conference preview | Bioworkshops will attend the Festival of Biologics 2025 in Basel
We look forward to connecting with industry leaders and sharing our expertise in complex antibody development and manufacturing. Come meet us to discuss how our CDMO solutions can accelerate your biologics journey.Bio Share | TsAb: Innovations and Challenges of Next-Generation Antibody Therapies
At present, 18 bispecific antibodies (BsAbs) have been approved worldwide, with the majority indicated for oncology.Towards Comprehensive Characterization of Antibody Drugs: Focus on Physicochemical Properties
The monoclonal antibody (mAb) technology was invented in 1976 by Georges Köhler and César Milstein. Since then, it has undergone four major stages of development: murine, chimeric, humanized, and fully human antibodies.Platform Strategies for Complex Antibody Expression: From Vector Engineering to System-Level Optimization
As of July 2025, a total of 20 bispecific antibodies (#BsAbs) have been approved worldwide (Figure 1). The majority are indicated for cancer immunotherapy, while a smaller number target chronic inflammatory and hematological diseases.